In Hekma Clinical Research, Excellence is the axis of success, and Training is the key to achieve excellence. We have different training programs in clinical research and research methodology
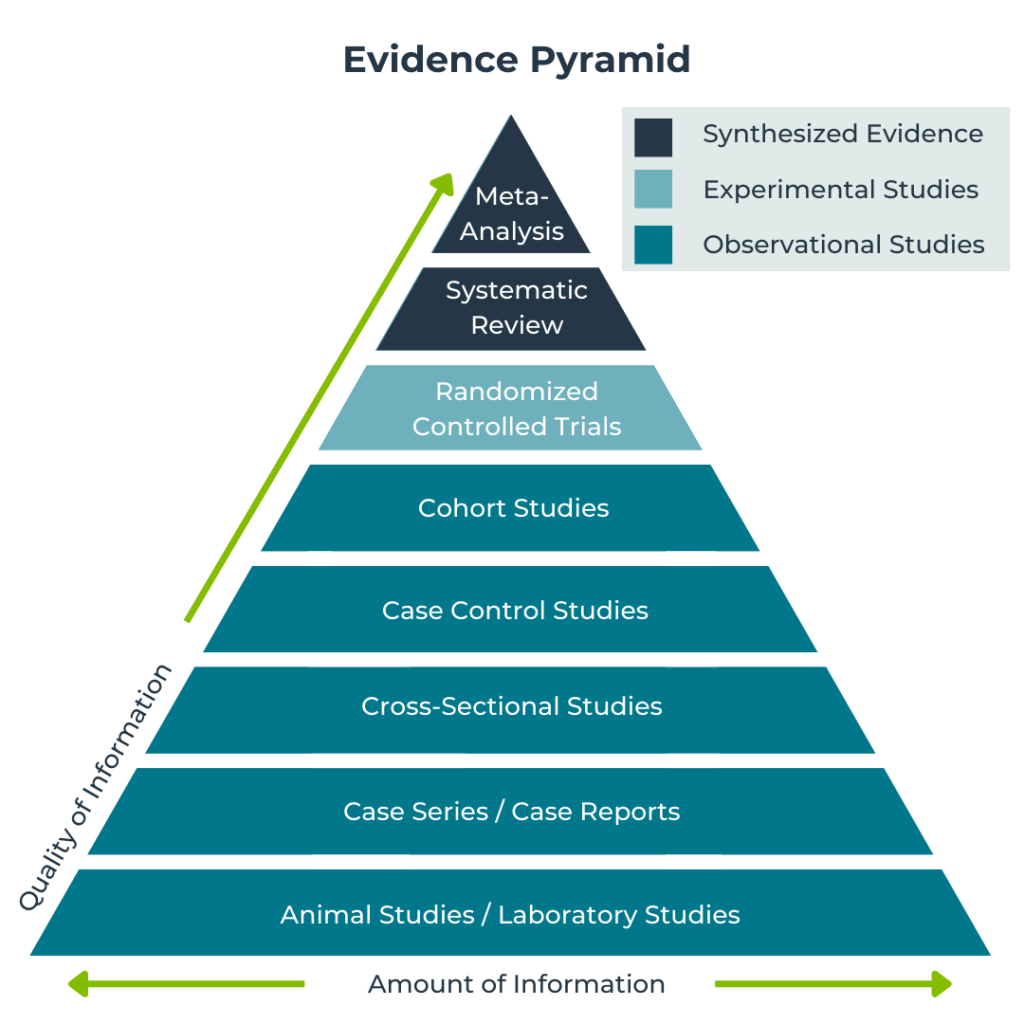
Clinical Research Training Pyramid
The PYRAMID of clinical research training is an educational system for clinical research that is based on an integration of education with research. The most important parts of the curriculum are training in data processing, problem-solving skills, project management and GCP.
Continuous methodology consultations, grants for proposals of participants, periodical exams and ranked diplomas for each of the 4 major levels are some of the unique features of this delicately devised system.
Good Clinical Practice (GCP) Workshop: What is GCP?

In recent years, all clinicians who wish to conduct or monitor clinical trials are required to have approved GCP training. We consider GCP training as a prequalification for clinical investigators and in the near future it would be a necessary part of clinical research. Hekma Clinical Research as the pioneer of clinical research industry is ready to manage GCP courses and would announce a GCP e-learning course soon. Clinicians and clinical investigators, staff of research centers and hospitals are invited to take part in these useful courses.
CONSORT Workshop: What is CONSORT?
CONSORT Statement is an evidence-based, minimum set of recommendations for reporting RCTs. It offers a standard way for authors to prepare reports of trial findings, facilitating their complete and transparent reporting, and aiding their critical appraisal and interpretation. Now many journals ask the authors to submit their manuscript according to the CONSORT Statement. You may wish to take part in a training course to learn the concepts of CONSORT Statement and its 22-items checklist necessary to submit your Trial manuscript. Therefore you can register for this workshop. Hekma Clinical Research is among the rare organizations to offer this learning opportunity in the region.
RCT Registry Workshop: Why RCT Registry?
The effectiveness of medical interventions should be based on the results of all properly conducted clinical trials, whether the trials have been published. Registration of clinical trials at their inception, in widely available registries, makes it possible for all stakeholders to take unpublished trials into account when summarizing the evidence for an intervention's effects. Now journal editors widely believe in the necessity of RCT registry and ask the authors of submitted manuscripts to present the registration no. of their study. So, investigators need to know the major registries and conditions and ways to register their studies before beginning or soon after first patient recruitment. Hekma Clinical Research gives you this opportunity to take part in such a workshop.

Clinical Research Study Group
Clinical Research Study Group (CRSG) is a study group for volunteers who wish to know more about pharmaceutical sciences and then join Hekma Clinical Research team. The educational curriculum of this group contains 4 major steps:
Step 1: RCT Design
Step 2: GCP Training
Step 3: Ethical Considerations
Step 4: Pharmaceutical Concepts of Clinical Studies
After passing these steps and when we ensure that the participants are well qualified, they may take part in conducting and monitoring the clinical studies.
